Marc Rothenberg, MD, PhD, is the director of the Cincinnati Center for Eosinophilic Disorders (CCED) at Cincinnati Children’s Hospital Medical Center and principal investigator of the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). In his clinical practice and research work, he focuses on solving the mysteries of allergic inflammatory diseases, especially eosinophilic gastrointestinal disorders (EGIDs). Here, he shares his inspirations for studying rare diseases, recent discoveries, and future plans.
What inspired you to become a physician and a researcher in the rare disease space?
Rare diseases are compelling because they are understudied by definition compared to more common diseases, and there is thus a potentially great impact of solving scientific questions. While I was completing my PhD, I started off looking at a rare disease called hypereosinophilic syndrome. It was very rewarding to work on a problem that was not well understood. As I got to know the limited number of patients that had the disease, I saw how much they were counting on me to make a difference.
Eventually, I was recruited to Cincinnati Children’s to develop allergy and immunology research. In 2001, I set up the CCED, which was the first center anywhere in the world to focus solely on eosinophilic disorders. Today, it has become exemplary for dozens of eosinophilic centers around the world.
Over time, we developed a center that is characterizing and bringing attention to these diseases. This center inspired others, and now we are a global network of centers across the world. Eosinophilic disorders still remain rare diseases, but we have developed enough information that they are featured on the covers of major medical magazines, talked about in medical schools, and included in medical textbooks. These diseases are now readily recognized by medical students, physicians, and pediatricians in particular.
"The more we understand, the better we can develop ways to treat these diseases."
What has been your biggest “aha” moment as a scientist?
- The FDA-approved drug alpha-1 anti-trypsin (A1AT) reverses damaging inflammation in an animal model of eosinophilic esophagitis
- Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease
- Mepolizumab as a treatment for patients with hypereosinophilic syndrome
The “aha” moments have been when we put together pieces of a puzzle—not knowing anything about what the puzzle would look like—and finally inserting the last piece to get the answer. Often, the discovery is not a pathway or mechanism that is super complicated. It is usually logical, integrated, and cooperative with our general view of the immune system. In other words, the piece of the puzzle fits right in!
There are certainly a lot of pieces still missing because we have more to discover—understanding the mechanisms of disease is a lot to uncover. But when you do uncover them, you find out that it is a really beautiful system. It is encouraging because the more we understand, the better we can develop ways to treat these diseases.
"Some of the greatest ideas come from patients and families."
You put a lot of effort into communicating research updates with patients and advocacy groups. Why do you think this is important?
I learn from everyone. Some of the greatest ideas come from patients and families. My research team is holistic—we come from diverse multidisciplinary and personal backgrounds. This includes people who are suffering from the diseases that we study.
That is how we started our partnership with the patient advocacy groups. Parents of patients reached out to us, and we began an interaction that was mutually inspiring to help families get involved and further our research. Together, we have advanced the field and helped patients around the world.
It is all part of our mission—communicating, learning, and educating. We have an obligation to communicate our findings to the outside world at all levels. By sharing our research in the public domain, such as through the EGIDExpress, we are able to advance the field even further. When people ask me whether a cure is coming, I can say that I am working on it, and I am confident that the work that we do will contribute to the cure.
How did CEGIR come together and what role has it played in your work?
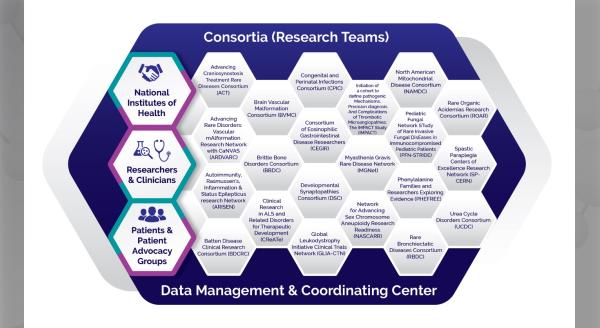
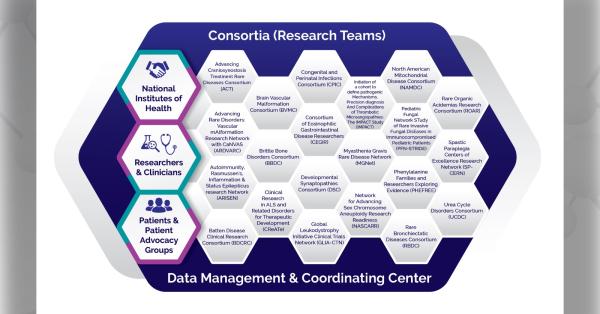
CEGIR was funded in 2014 by the Rare Diseases Clinical Research Network (RDCRN). This has been a very special opportunity to bring together not only experts who are interested in these diseases, but also partners who are diverse stakeholders, particularly patient advocacy groups that are key to our work.
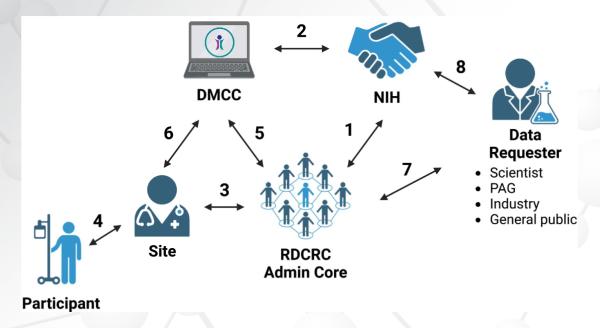
We very much appreciate the high-quality research enterprise associated with the RDCRN. The Data Management and Coordinating Center (DMCC) and their ability to facilitate research helps us manage the data and make sure that it is pristine. Many elements go into increasing the impact of our findings. Since 2014, we have published a number of articles, and we have many more ahead. These developments have made it an exciting time.
"I believe that the cure is going to come from taking a wide approach."
What do you see ahead for CEGIR and your rare disease research?
Our goal is to continue to have a bigger impact on patients. We need to treat all patients, not just those who have one particular type of disease. We are now moving from the esophagus down to the stomach, from the intestine into the colon—we aim to treat the specific diseases that affect these areas using approaches of precision and personalized medicine.
We still need to get drugs approved by the FDA for these diseases. My team will not rest until we find these treatments. We will continue our fundamental research to understand the basis of these diseases.
I strongly support the interaction of all facets of research—basic fundamental studies, genetics, translational systems, and clinical trials. I believe that the cure is going to come from taking a wide approach.
Visit the Rothenberg CURED Lab at Cincinnati Children’s.
The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). CEGIR is funded under grant number U54AI117804 as a collaboration between NCATS, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).