Each month, we share summaries of recent Rare Diseases Clinical Research Network (RDCRN) grant-funded publications. Catch up on the latest RDCRN research below.
Jump to:
- Brain Vascular Malformation Consortium (BVMC)
- Brittle Bone Disorders Consortium (BBDC)
- Developmental Synaptopathies Consortium (DSC)
- Genetic Disorders of Mucociliary Clearance Consortium (GDMCC)
- Lysosomal Disease Network (LDN)
- Porphyrias Consortium (PC)
- Urea Cycle Disorders Consortium (UCDC)
Brain Vascular Malformation Consortium (BVMC)
Cerebral cavernous malformations (CCMs) are collections of small blood vessels in the brain that are enlarged and irregular in structure, leading to altered blood flow. While approximately 25 percent of individuals with CCMs never experience any related medical problems, other people with CCMs may experience serious symptoms such as headaches, seizures, paralysis, hearing or vision deficiencies, and cerebral hemorrhage.
In addition to lesions in the brain, familial cases have lesions present on the skin. Interestingly, similar appearing skin lesions have been reported in another inherited vascular disease called capillary malformation-arteriovenous malformation (CM-AVM), which is caused by mutations in RASA1 and EPHB4.
In this study, researchers investigated whether common variants in the EPHB4 and RASA1 genes are associated with familial CCM disease severity, including intracranial hemorrhage (ICH), total lesions, and large lesion counts. They found that EPHB4 variants were not associated with CCM severity, but a common RASA1 variant may be associated with ICH and large lesion count.
These findings could improve understanding of the natural history of CCM, leading to better predictions of disease course and new medical therapies for treatment.
Vascular malformations are growths composed of blood vessels involving arteries, veins, capillaries, and lymphatics. Patients with vascular malformations in the central nervous system may experience a range of debilitating or life-threatening symptoms including seizures, headaches, and increased risk of cerebral hemorrhage.
Due to their inaccessible location, these malformations are difficult to monitor and treat. Therefore, biomarkers from a non-invasive tissue source, such as blood, may aid in predicting disease severity and outcomes.
In this study, researchers compared circulating biomarker levels in plasma from patients with sporadic brain arteriovenous malformation (BAVM), familial cerebral cavernous malformations (CCM), and hereditary hemorrhagic telangiectasia (HHT). They found that biomarkers may be unique to each type of vascular malformation, indicating potential usefulness in assessing phenotypic traits of vascular malformations.
Brittle Bone Disorders Consortium (BBDC)
COPB2 loss of function causes a coatopathy with osteoporosis and developmental delay
Vesicle coat proteins help cells sort and transport or “traffic” proteins and lipids. Pathogenic variants (mutations) in genes that encode subunits of coat complexes called coatomers are believed to contribute to a number of genetic disorders called coatopathies that can affect the skeletal and central nervous systems.
In this study, researchers examined loss-of-function variants in the gene COPB2, which encodes for a protein in the coatomer complex, in six individuals from five unrelated families who have osteoporosis or osteopenia (brittle bones that may fracture easily) and variable degree of developmental delay. Researchers also used zebrafish and mouse models to further study the effect of COPB2 deficiency on collagen trafficking because of the critical role of collagen secretion in bone development.
The authors conclude that COPB2 haploinsufficiency (meaning only 50% of the normal active form of a particular protein is expressed) is a cause of this form of coatopathy. They also tested ascorbic acid supplementation as a potential treatment and found that it had a beneficial effect on animal models.
Women with rare diseases considering pregnancy often lack data regarding outcomes, specific risks, and management strategies. The Brittle Bone Disorders Consortium established an Osteogenesis Imperfecta Pregnancy Registry to collect data on pregnancy, maternal, and neonatal outcomes in women with osteogenesis imperfecta (OI), or brittle bone disease.
A total of 132 participants with OI completed a cross-sectional, survey-based study. Of respondents, 34% had moderate to severe OI. Researchers compared self-reported information on pregnancy and maternal and neonatal outcomes of women with OI with data on the general population, referenced by literature-based standards.
Results indicated that women with OI had higher rates than the general population of diabetes in pregnancy, cesarean delivery, need for blood transfusion, and fractures before or after delivery. Individuals with moderate or severe OI reported higher maternal hospitalization and delivery rates than those with mild OI. Babies born to women with OI had higher rates of neonatal intensive care unit admissions and higher neonatal mortality, regardless of neonatal OI status.
Study authors say that patients and providers should be aware of these findings, particularly the need for blood products (hemorrhage) and the increased rate of fractures, low birthweight infants, and neonatal mortality. They suggest that survey results can support both preconception counseling and proactive measures to reduce harm and recognize modifiable risk factors related to pregnancy.
Developmental Synaptopathies Consortium (DSC)
PTEN hamartoma tumour syndrome: what happens when there is no PTEN germline mutation?
More than 400 hereditary cancer syndromes have been described to date and account for 5-10% of all cancers. PTEN hamartoma tumour syndrome (PHTS) is an umbrella term for subsets of four syndromes associated with germline (inherited) PTEN mutations. However, many patients with phenotypes similar to those in PHTS do not carry germline PTEN mutations.
This paper reviews gene discovery efforts over the last decade to identify alterations in cancer-predisposing genes in order to facilitate gene-informed molecular diagnosis, cancer risk assessment, and gene-specific clinical management. Authors conclude that validating these discoveries is critical to bringing these patients specific gene-informed risk assessment and subsequent management.
Genetic Disorders of Mucociliary Clearance Consortium (GDMCC)
This case report highlights the difficulty distinguishing primary ciliary dyskinesia (PCD) from primary immunodeficiency (PID) with particular emphasis on the potential overlap in nasal nitric oxide levels between the two disorders.
An 11-year-old female with history of chronic wet cough, chronic nasal congestion, and recurrent lower respiratory tract infections was referred for evaluation of possible PCD. Her nasal nitric oxide level was low (9.8 nL/min) and remained low at follow-up one year later (17.5 nL/min). Ciliary ultrastructure on transmission electron microscopy (TEM) was normal and PCD genetic testing was unrevealing, but given her clinical history and low nasal nitric oxide, she was classified as “probable PCD.”
Later, at the age of 21, she became acutely ill, was diagnosed with hemophagocytic lymphohistiocytosis, and died secondary to this. She was subsequently found to have a pathogenic variant in GATA2 resulting in GATA2 deficiency, a syndrome characterized by immunodeficiency and predisposition to myelodysplastic syndrome.
This case illustrates how individuals with PID can also have persistent low nasal nitric oxide levels. In those with suspicion for PCD but without a definitive diagnosis (confirmed by either PCD genetic testing and/or abnormal ciliary ultrastructure on TEM), clinicians should consider genetic testing for PID.
Lysosomal Disease Network (LDN)
A diagnostic confidence scheme for CLN3 disease
CLN3 disease is an inherited disorder that primarily affects the nervous system. After 4 to 6 years of normal development, children with this condition develop vision impairment, intellectual disability, movement problems, speech difficulties, and seizures, which worsen over time.
Researchers seeking to improve diagnostic methods for CLN3 disease used genotype and phenotype data from an ongoing natural history study to develop a hierarchical diagnostic confidence scheme with three major classes: Definite, Probable, or Possible CLN3 disease. An additional level, CLN3 Disease PLUS, includes individuals with CLN3 disease plus an additional disorder that substantially affects the phenotype. They used the scheme to classify individuals and then performed a blinded reclassification to assess the reliability of this scheme. Test-retest reliability showed 96% agreement.
Authors conclude that their diagnostic confidence scheme for CLN3 disease appears to be effective and has implications for clinical research in CLN3 and other rare genetic neurodegenerative disorders.
Porphyrias Consortium (PC)
Porphyrias are rare disorders caused by an abnormality in the heme production process. Heme enables our blood cells to carry oxygen and helps break down chemical compounds in the liver.
Erythropoietic protoporphyria patients experience a build-up of protoporphyrin in the bone marrow, red blood cells, blood plasma, skin, and eventually liver. This buildup can cause extreme sensitivity to sunlight, liver damage, and other problems.
The protein CLPX (caseinolytic mitochondrial matrix peptidase chaperone subunit X) promotes heme synthesis. Researchers seeking to better understand the ways CLPX regulates heme synthesis in red blood cells undertook genomic studies in yeast, zebrafish, and mouse models.
They found that CLPX mutations may cause anemia and porphyria via dysregulation of ALAS, FECH, and PPOX activities, as well as of iron metabolism. They conclude that unraveling the complexities of CLPX function will be key for designing therapies for these rare diseases.
Urea Cycle Disorders Consortium (UCDC)
In the liver, the enzymes argininosuccinate lyase (ASL) and argininosuccinate synthase 1 (ASS1) are required to convert waste-nitrogen to urea. Loss of activity for either enzyme causes argininosuccinate lyase deficiency and citrullinemia type 1, respectively. These two disorders are a subset of the classical inborn errors of metabolism called urea cycle disorders (UCD), characterized by episodes of hyperammonemia.
ASL deficiency can also result in impaired nitric oxide (NO) synthesis, decreased tyrosine hydroxylase (TH) activity, and low dopamine and norepinephrine levels in the neuronal cells. Both dopamine and norepinephrine are important neurotransmitters, and their deficiency has been associated with neurodegenerative disorders, including Parkinson's disease.
In this study, researchers used a mouse model with loss of ASL in catecholamine neurons to test the hypothesis that decreased activity of ASL and TH would contribute to neurodegeneration. They found that neuronal loss of ASL results in catecholamine deficiency, in accumulation and formation of tyrosine aggregates, in elevation of α-synuclein, and phenotypically in motor and cognitive deficits.
Study authors say their data point to a potential metabolic link between accumulations of tyrosine and seeding of pathological aggregates in neurons as initiators for the pathological processes involved in neurodegeneration. They suggest that regulating NO levels may be beneficial for the treatment of catecholamine-related neurodegenerative disorders.
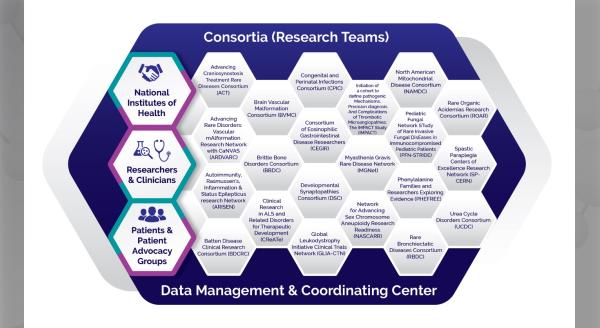
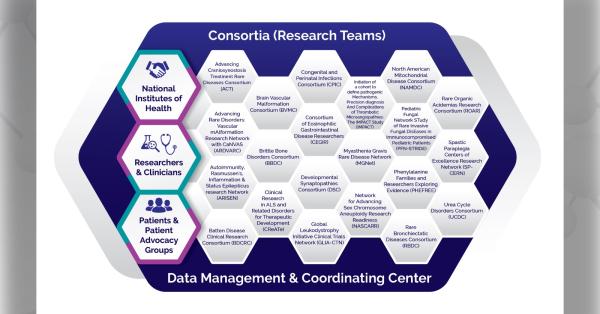
The Rare Diseases Clinical Research Network (RDCRN) is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). Now in its fourth five-year funding cycle, RDCRN is a partnership with funding and programmatic support provided by Institutes, Centers, and Offices across NIH, including the National Institute of Neurological Disorders and Stroke, the National Institute of Allergy and Infectious Diseases, the National Institute of Diabetes and Digestive and Kidney Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, the National Heart, Lung, and Blood Institute, the National Institute of Dental and Craniofacial Research, the National Institute of Mental Health, and the Office of Dietary Supplements.