Marc Rothenberg, MD, PhD, the principal investigator of the Consortium of Eosinophilic Gastrointestinal Diseases (CEGIR), and Shawna Hottinger, MS, ELS, of Cincinnati Children’s Hospital Medical Center, report here on “Impressions and Aspirations from the FDA GREAT VI Workshop on Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis and Perspectives for Progress in the Field.”
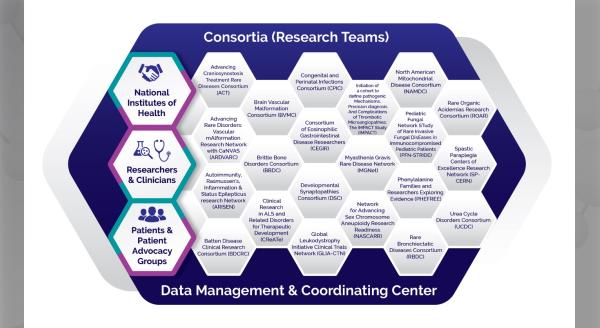
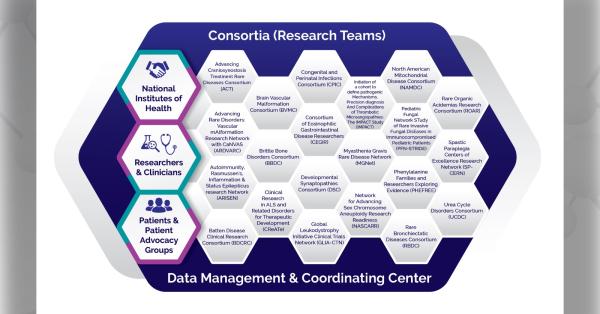
The U.S. Food and Drug Administration (FDA) hosted a workshop on July 21, 2021 to discuss the disease characteristics, natural history, and endpoints to assess treatment benefit in patients with eosinophilic gastrointestinal disorders (EGID) beyond eosinophilic esophagitis (EoE). Researching these disorders is the mission of the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR), a national research network of the National Institutes of Health-funded Rare Diseases Clinical Research Network (RDCRN).
Eosinophilic gastrointestinal diseases (EGIDs) are rare allergic conditions of the gastrointestinal tract, with eosinophilic esophagitis (EoE) being the most commonly studied. Other types of EGIDs, such as eosinophilic gastritis, eosinophilic enteritis, and eosinophilic colitis, are understudied relative to EoE.
This workshop provided a forum for open discussion among stakeholders—medical professionals (including their societies and research groups), FDA representatives, an industry representative, and a patient representative—to facilitate drug development. Experts in many disciplines related to EGID, including allergy, immunology, epidemiology, gastroenterology, and pathology, and both adult and pediatric clinicians contributed. The full report on the FDA workshop is available here or view a meeting recording here.
Importantly, diagnosis of EGID is often delayed, and EGIDs have no U.S. Food and Drug Administration (FDA)-approved treatments.
Experts in many disciplines related to EGID (e.g., allergy, immunology, epidemiology, gastroenterology, pathology), adult and pediatric clinicians, and stakeholder representatives (FDA, industry, patient) contributed to this workshop. We wish to share with you a few of the recently published insights of the meeting and perspectives on moving the field forward toward FDA-approved treatment; for the full discussion, including further information on diagnostic delay circumstances and solutions and nomenclature, read the article.
Several key points that arose from the meeting include:
- Diagnosis of non-EoE EGID can be challenging, as eosinophils are resident cells during homeostatic healthy conditions in the non-esophageal portions of the gastrointestinal tract. Nevertheless, eosinophil thresholds for diagnosis, especially in patients with eosinophilic inflammation of the stomach and duodenum, and more comprehensive disease manifestation assessments are steadily being refined to permit the assessment of meaningful benefit in clinical practice and in clinical trials.
- Standardized nomenclature for clinical and research purposes for non-EoE EGIDs is fundamental for further progress and is being developed.
- The non-EoE EGID field is expanding, and data related to diagnosis, natural history, and pathogenesis are rapidly emerging and transforming clinical practice.
- As chronic diseases associated with substantial morbidity, health care utilization, and poor quality of life, EGIDs are in desperate need for efficacious and FDA-approved drugs.
- Potential differences in assessment standards, diagnostic and eligibility criteria, and outcome measures between clinical practice and those suitable for regulatory purposes to support drug development should be considered prior to trial initiation to inform design and promote the interpretability of the results to support the assessment of clinical benefit.
Below* are examples of challenges and progress to defining clinical benefit for non-EoE EGIDs:
| Challenges | Progress |
| Lack of clinical consensus diagnostic criteria | Consensus criteria are currently being developed for non-EoE EGID (CEGIR initiative). |
| Heterogeneous nomenclature | Harmonized nomenclature is currently being developed (CEGIR initiative). |
| Differences in clinical practice and regulatory assessment standards and outcome requirements | An integrated approach is recommended. |
| Lack of regulatory or drug development precedent | Collaboration of key stakeholders and open dialogue, such as this FDA GREAT VI Workshop, are essential. |
| Rare diseases with few patients available to participate (although disease recognition is increasing) | Collaborative research involving multiple sites and partnering with patient advocacy groups helps identify, recruit, and maintain patients in research (practices adopted by CEGIR). |
| Multi-center, multi-country trials required to enroll sufficient patients | Open dialogue, collaboration, registries, and meetings across diverse geographic, demographic, racial, and ethnical groups are underway (e.g., patient advocacy groups, European Eosinophil Society [EurEoS], The International Gastrointestinal Eosinophil Researchers [TIGER]). |
| Pediatric-specific considerations | It is important to realize that children and adults can require unique considerations, which are discussed through collaborative research endeavors and networks that include both adult and pediatric clinicians and researchers, such as CEGIR (involvement of patient advocacy groups; e.g., American Partnership for Eosinophilic Disorders [APFED], Campaign Urging Research for Eosinophilic Diseases [CURED], Eosinophilic Family Coalition [EFC], ausEE, EOS Network) |
Among the next steps that would benefit the non-EoE EGID field, a few relate specifically to clinical trials and FDA approval of drugs for EGID indications: 1) Define clinical benefit in clinical trials through collaborations with patients, patient advocates, researchers, clinicians, industry, regulatory agencies, and other stakeholders; 2) Incorporate frequent and early interaction with the FDA for drug development and the prospective design and use of anchor-based analyses to promote the detection and characterization of clinically meaningful change and facilitate interpretation of results across drug development programs from even the early stages of drug development; 4) Develop consensus clinical trial endpoint assessment criteria that are complementary to diagnostic criteria and clinical presentation for each non-EoE EGID to facilitate design and generalizability of findings for clinical trials; and 5) Develop FDA-approved drugs for EGID indications; current EGID treatments are limited to off-label uses or clinical trials.
Below* are examples of outstanding questions and progress in the non-EoE EGID field, many of which have been enabled by CEGIR:
| Question | Progress |
| How do we interpret eosinophil number in tissues that have eosinophils during homeostasis? How can we improve diagnosis for non-EoE EGID? | Consensus criteria are being established in a consensus process led by CEGIR. |
| Is the number of eosinophils a relevant parameter or, for instance, may localization of the eosinophilia or other histologic or molecular findings be more informative for disease assessment and outcomes and/or therapeutic endpoint assessment? | Histology Scoring Systems (HSS) that take into account a number of parameters in addition to eosinophils are being developed. Likewise, endoscopic scoring systems have been (e.g., EoG-EREFS) and are being developed. Molecular and cellular dissection of disease pathogenesis is developing a framework for disease biomarkers. Furthermore, patient-reported outcome (PRO) metrics are being developed and have been used in EoG/EoD clinical trials; their co-implementation with clinical care is a focus area. |
| Why do non-EoE EGID have lower prevalence than EoE? Are they underdiagnosed? | Prevalence studies are ongoing and have suggested that a combination of factors, including increased disease recognition and the allergy epidemic, are contributing to increased diagnosis of non-EoE EGID. |
| How do non-EoE EGIDs, IBS, DBGI (formerly FGID) relate? What does this mean for our understanding of non-EoE EGID? | A detailed analysis of eosinophil levels and type 2 immunity is currently underway; disease overlap may be present in some patients. |
| Why are some tissues affected and not others? | This is a cardinal question, and detailed studies of EoE suggest that tissue-specific, genetically defined pathways and susceptibility are involved. |
| Are there differences between resident and infiltrating eosinophils and their role in disease initiation, perpetuation, resolution, and/or recurrence? | Fundamental studies about eosinophil heterogeneity are revealing multiple populations of eosinophils, some with potential helpful properties (e.g., regulatory eosinophils). The role of eosinophil heterogeneity in EGID is an outstanding question. |
| Do the affected areas represent distinct diseases in a continuum or a spectrum? | Evidence is emerging that distinct tissue involvement in non-EoE EGID may represent a disease continuum, but this is a debated topic. |
| How do we separate functional dyspepsia, DBGI (e.g., IBS), and EGID? | In most clinical cases, a thorough histologic and pathologic evaluation will lead to the diagnosis of EGID. Moreover, clinicians with familiarity with non-EoE EGID clinical manifestation and appropriate medical workup are able to recognize and differentiate these diseases for clinical and research purposes. Detailed cellular, molecular, and neuro-immunologic characterization of these diseases will likely facilitate future diagnosis and patient phenotyping and endotyping. |
| What is the EoC disease course and does it differ from gastric and enteric EGID disease courses? What underlies EoC’s lower association with atopy relative to other non-EoE EGID? | Preliminary studies suggest a distinct etiology and disease course for EoC; more studies are needed. |
| What are appropriate endpoints to assess a treatment’s clinical benefit for each non-EoE EGID? | A series of broad outcome metrics spanning histologic, endoscopic, molecular, and clinical features are being developed. This is a focus area of CEGIR. |
| How can the development of approaches and assessments for “clinical benefit” for clinical trials and “meaningful benefit” for clinical practice be complementary? | Increasing dialogue between key stakeholders, including patients, researchers, and FDA and industry representatives, will be key in this regard. |
| What are appropriate endpoints to assess a treatment’s clinical benefit for each non-EoE EGID? How can non-invasive and QOL assessments be developed and/or validated for meaningful benefit for clinical practice? | A series of validated endpoints involving clinical, endoscopic, histologic, and molecular features are being developed and will likely prove to be valuable. |
*Tables reprinted from the Journal of Allergy and Clinical Immunology with permission from Elsevier. Rothenberg ME, Hottinger SKB, Gonsalves N, Furuta GT, Collins MH, Talley NJ, Peterson K, Menard-Katcher C, Smith M, Hirano I, Genta RM, Chehade M, Gupta SK, Spergel JM, Aceves SS, Dellon ES. Impressions and aspirations from the FDA GREAT VI Workshop on Eosinophilic Gastrointestinal Disorders Beyond Eosinophilic Esophagitis and Perspectives for Progress in the Field. J Allergy Clin Immunol. 2021 Dec 22:S0091-6749(21)02671-3. doi: 10.1016/j.jaci.2021.12.768. Epub ahead of print. PMID: 34953790.
The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). CEGIR is funded under grant number U54AI117804 as a collaboration between NCATS, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).