The Office of Rare Disease Research (ORDR) estimates that 7000 rare diseases affect 25-30 million people with a rare disease in the US - 8-12% of the US population. However, for those of us who care for patients with a rare disease and for the patients themselves, the disease is no longer rare-it is a constant part of the patient's life and the life of their families. Moreover, for the "interesting" patient with a rare disease, being "an interesting case" to physicians may make the patient feel that their physicians are in league with their "interesting" disease. Discovering treatments for each patient with each disease - and making each patient feel that they are being cared for is a monumental challenge.
The Strengths of the Rare Disease Clinical Research Network:
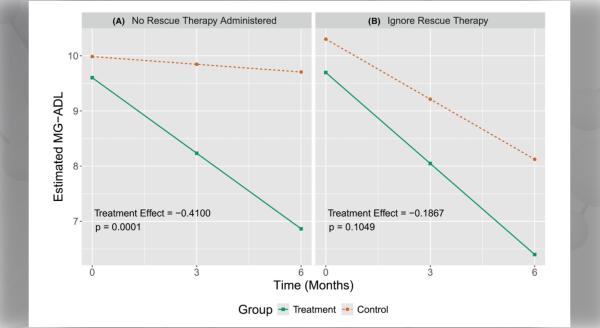
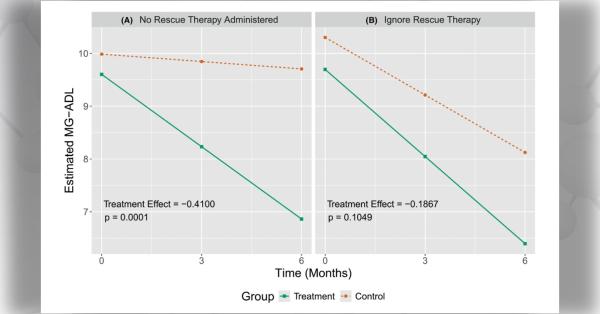
- The Data Management Coordination Center (DMCC) under Dr. Jeffrey Krischer, PhD, at the University of South Florida has brought unanticipated strengths to CINCH. As partners in navigating the complex regulatory environment that patient-oriented researchers work within, they have been resourceful in accelerating protocol development, approval and implementation as well as managing and analyzing data. DMCC investigators have also learned from each RDCRN consortium to the benefit of others. CINCH's collaboration with the DMCC rapidly developed and implemented a Patient-Reported Outcome Measure (PROM) for each protocol. These PROM now form the primary outcome for each of CINCH's clinical trials in our target diseases.
- NINDS, NCRR and ORDR Support: CINCH has benefitted from working hand in glove with National Institute of Neurological Disease and Stroke (NINDS) Program Director of Extramural Research Dr. Randall Stewart, PhD, as well as ORDR Liaison neurologist, Dr. John Ferguson. ORDR's Dr. Stephen Groft has been an advisor and supporter from the outset. We have been able to hold 8 international meetings with NIH R-13 support. Other support and advice regarding shared resources have come from NINDS, ORDR and the National Center for Research Resources (NCRR) - especially through Rochester's Clinical and Translational Science Award (CTSA).
- The RDCRN and the FDA: In keeping with the all-important goal of bringing new treatments to patients with rare diseases, the RDCRN has promoted interaction with the Food and Drug Administration's Rare Disease experts. CINCH has agents in clinical trials for our diseases and has already worked with the FDA to obtain Orphan Product Designation for two agents. This step will be important once we obtain regulatory approval, to then be able to have the new treatment marketed by our industry partners for "our" diseases.
- RDCRN Pilot Study Funding: Funding of London, UK. CINCH site-principal investigator Prof. Michael Hanna, and CINCH trainee Emma Matthews enabled CINCH to find new mutations in the dihydropyridine receptor in patients with periodic paralysis. Funding of a pilot trial of treatment for EA-2 was obtained only to have the drug prove to be unavailable for three years. Fortunately, CINCH was able to secure FDA Orphan Product grant funding once the agent received regulatory approval for another disease.
- Patient Advocacy Groups: Patients with rare diseases worked to attract attention to the need of each disease for research focused on treatment. Their work continues. CINCH has had the support of the Muscular Dystrophy Association (MDA) for work on Andersen-Tawil syndrome and non-dystrophic myotonia with MDA's Annie Kennedy working as a partner. The Periodic Paralysis Association with Linda Feld has been full partners in our recruitment efforts. The National Ataxia Foundation with Sue Hagen has been a proactive supporter of our episodic ataxia work.
- Learning from Other Consortia: The RDCRN has brought together like-minded clinician-scientists focused on meeting the challenges of rare disease research. As examples, CINCH has learned patient recruitment strategies such as Dr. Alan Percy's (Angelman, Rett, Prader-Willi Syndromes Consortium) travelling Rett Syndrome clinics and then developed a similar plan for our patients with episodic ataxia; Dr. Mark Batshaw (Urea Cycle Disorders Consortium) and Dr. Peter Merkel (Vasculitis Clinical Research Consortium) shared strategies for recruiting and mentoring trainees; Arthur Beaudet was the inaugural lecturer for our annual Rare Disease Lectureship.
- Rare Diseases Visiting Lectures: CINCH has initiated a program to bring visiting professors to universities within the consortium. These experts provide lectures on the rare disease research and increase awareness of these disorders. We make the lectures widely available, and they are targeted not only to neurology, but all departments on our campuses.