Lymphangioleiomyomatosis (LAM) is a rare, metastatic neoplasm of women in which the lung becomes infiltrated with smooth muscle cells bearing mutations in tuberous sclerosis complex (TSC) genes. The source of the cells is not known, but it is clear that both lymphatic and hematogenous spread occur. Cystic lung destruction ensues, most likely through protease-driven matrix degradation and disordered lymphangiogenic remodeling induced by the invading LAM cells. Lung function declines at rates that are 2-5 fold faster than the natural rate in adults, and most patients require supplemental oxygen by the tenth year after diagnosis. In patients who present with shortness of breath, the median time from diagnosis to death or lung transplant is approximately 10 years.
The TSC gene mutations that cause LAM result in constitutive activation of the mTOR pathway that controls cell growth. Sirolimus is a selective mTOR inhibitor which has been FDA approved for the prevention of renal transplant rejection since 2000.
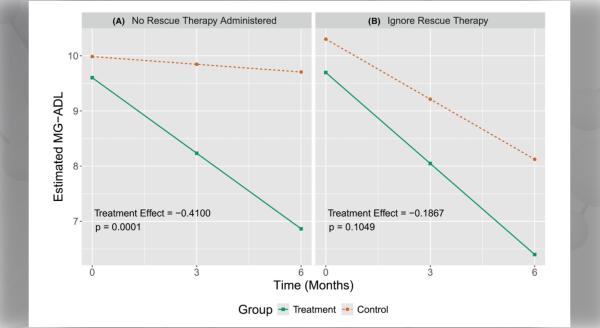
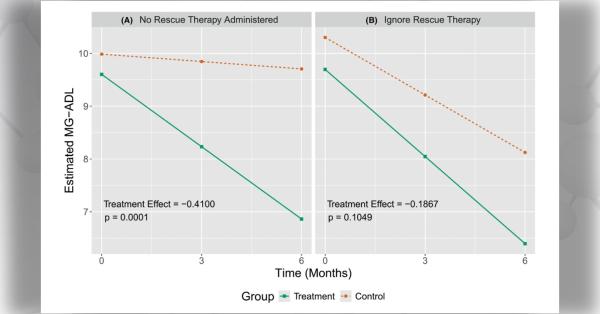
A double blind, randomized, Multicenter International LAM Efficacy of Sirolimus (MILES) trial was designed to test the hypothesis that sirolimus improves lung function in patients with LAM. MILES was conducted through the Cincinnati-based Rare Lung Disease Consortium (RLDC); a network of 10 domestic sites and 3 international sites in Canada and Japan. Of 111 patients consented, 89 were randomized to receive sirolimus or placebo for 1 year, followed by a 1 yr observation period off therapy. The primary endpoint was the difference between groups in the rate of change in slope of forced expiratory volume in 1 second (FEV1). The trial, reported in The New England Journal of Medicine this spring1, showed that sirolimus is a treatment for selected patients with LAM. Sirolimus stabilized lung function and resulted in improvement in some measures of functional performance and quality of life over one year on therapy. Lung decline appeared to resume when the drug was stopped.
The National Organization for Rare Disorders (NORD) awarded the Partners in Progress Award to MILES investigators and the LAM Foundation for demonstrating "how an effective partnership between scientific researchers and the patient community can drive progress in better understanding of rare diseases and possible treatments." This citation mirrors the founding principle of the RDCRN, as articulated by the leadership of the ORDR and the National Center for Research Resources.
MILES would never have occurred if the LAM Foundation had not spent the prior decade organizing and educating patients, and funding the basic and clinical research that formed the scientific basis for MILES. The Foundation also played important roles in patient recruitment and trial logistics during MILES, including acting as a conduit for making timely payments to sites for visit costs and to patients for travel costs. MILES would not have been feasible without the infrastructure that the RDCRN provided, including the resources to establish an effective network and to engage an experienced data center. The Cincinnati Children's Translational Research Trials Office functioned as the contract research organization, and navigated the complex waters of international trial regulations, FDA, Pharmaceutical and Medical Device Agency (PMDA) and the Canadian Health Products and Food Branch oversight, drug import and export, blinded dose adjustments and over 2000 adverse events. The investigators who conducted the trial were clearly motivated by a desire to offer their patients the hope for a future treatment, since the budget barely covered costs and the time commitment to participate was large for the small number of patients enrolled at most sites. Indeed, throughout this seven year journey, good will and noble intentions toward patients were the 'wind in our sails'. There were also many challenges that identified opportunities for improvement in future trials (Fig. 1), three of which are discussed below.
The regulatory hurdles to implementing a trial for a chronic, rare disease with an immunosuppressive therapy were daunting. There were seven revisions to the protocol over two years before the DSMB approved opening of the first site in Cincinnati. International and domestic regulatory and contracting issues across the network delayed opening of sites for up to two additional years beyond the Cincinnati site launch. The enrollment period was prolonged to the point that funding became depleted and the drug expired, requiring protracted negotiations with the donor company for an expensive remanufacturing effort. Monetary realities forced investigators to request a truncation of the observation period for some of the late enrolling patients, which limited the statistical power of some endpoints in the second year. Most of the delays could be traced to the many distractions and diverse priorities of government and academic institutions, which have a multitude of missions beyond clinical trials. In addition, regulatory bodies are composed of generous but overcommitted volunteers, who may be available only at intervals. It is clear that better systems which incorporate principles of urgency, efficiency, accountability and performance incentives are needed for the conduct of investigator initiated clinical trials in academic networks.
The $8.5 million that were raised in support for the trial came from a multitude of sources (Fig. 2). Of the $5 million in original U01 RDRCN funding which supported the aims of the RLDC grant, we estimate that about $3 million were consumed by the MILES trial. The Japanese Ministry of Health, Education and Welfare contributed $2 million, and an Orphan Drug Grant from the FDA approximately $1.1 million. Pfizer (formerly Wyeth) provided over $1 million in drug and $200,000 in visit costs. Remarkably, the LAM Foundation contributed over $500,000, which represented more than half of their net worth at the time of the donation. The Tuberous Sclerosis Alliance Rothberg Courage Award, and the Canadian Institutes of Health Research Award each added $200,000 to the bottom line. The Cincinnati Children's Hospital, the University of Cincinnati and the Adler Foundation all made sizeable donations of $40,000 to $100,000. In diseases too rare to be of interest to pharmaceutical firms, the willingness of government funding agencies, foundations, and academic centers to share trial ownership, and of international investigators to secure funding for site costs in their own countries, are critical to success.
A troubling and unresolved dilemma is that the Japanese patients, who assumed risk to participate in MILES, have had no access to the drug since the trial concluded. In Japan, the government is both the regulator and insurer of all pharmaceuticals requiring prescription, so that unless a drug is approved for a certain indication, it cannot be used. Although FDA approval would greatly accelerate PMDA approval of sirolimus in Japan, the manufacturer of sirolimus does not intend to pursue an indication for LAM because the drug is coming off patent. Pfizer does not intend to market sirolimus in Japan, but may well license the drug to a smaller pharmaceutical company. The LAM Foundation is currently investigating the feasibility of a Citizen's Petition to gain FDA approval for the drug in patients with LAM, primarily to facilitate access in other countries. Simultaneously, the Japanese MILES investigators are submitting an application to the PMDA, which in the absence of FDA indication may require additional studies before approval can occur. The pathways to FDA approval of drugs for rare diseases are not well lit, and should certainly not be shaped by market forces or evidence standards designed for more common and better resourced diseases. A clearly identified orphan drug liaison in the Office of Rare Disease Research or the FDA would be a great asset to clinical trialists interested in rare disease treatments.
I have heard from many MILES investigators, nurses, pharmacists, patients, and statisticians since the trial results were published. On behalf of the entire MILES Study Group, I would like to thank the Office of Rare Disease Research and the National Center for Research Resources for this very humbling and rewarding opportunity to seek a treatment for a rare lung disease.
References
1. McCormack FX, Inoue Y, Moss J, Singer LG, Strange C, Nakata K, Barker AF, Chapman JT, Brantly ML, Stocks JM, et al: Efficacy and safety of sirolimus in lymphangioleiomyomatosis. N Engl J Med 2011, 364(17):1595-1606.
Related Web Sites: