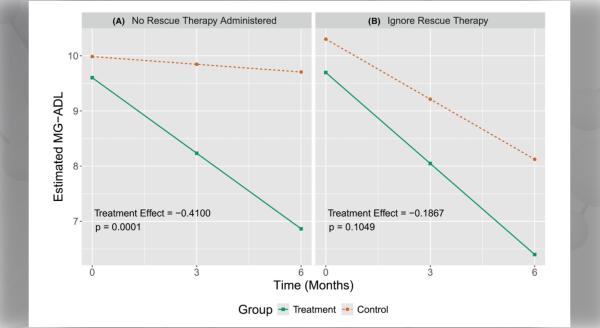
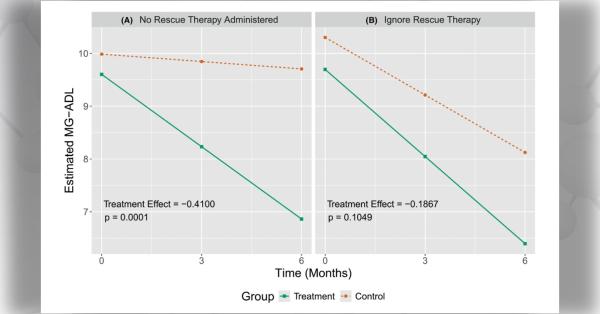
Eosinophilic gastrointestinal diseases (EGIDs) are a group of chronic immune system disorders in which a type of white blood cell (eosinophils) build up in the gastrointestinal tract, causing inflammation or injury. Patients with EGIDs can experience difficulty swallowing and eating, stomach pain, nausea, acid reflux, loss of appetite, diarrhea, weight loss, and poor growth.
To learn more about EGIDs, the Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) is conducting a natural history study, “Outcome Measures for Eosinophilic Gastrointestinal Diseases across Ages (OMEGA).” The team is exploring the correlation between patients’ symptoms and tissue samples to help reveal clues about the disease, as well as help guide diagnosis and treatment plans.
Here, lead investigator Glenn Furuta, MD, and 12-year-old EGID patient Tripp Sandstrom share more about the study and what it’s like to participate.
What are we learning about EGIDs from this study?
Dr. Furuta: There is very little data on the natural history of EGIDs, and this information is critical because of the need to develop clinical outcome metrics for clinical trials. In this study, we are collecting clinical data about the symptoms, quality of life, endoscopic features, and histology of patients with all different EGIDs (eosinophilic esophagitis, eosinophilic gastritis, eosinophilic enteritis, and eosinophilic colitis) to understand what these features are at the time of diagnosis and how they change over time.
How and why did you become involved with this study?
Sandstrom: My diagnosis is eosinophilic esophagitis (EOE). I stay away from milk as this triggers my EOE, but I can now have baked milk. I became involved with this study when I was first diagnosed at the age of four at Children's Hospital Colorado. We had a lady come and talk to us about this study as they were trying to find out more about it and asked if we wanted to be a part of it. I decided to join to try to find the cause of EOE and ways to help cure it.
What makes this study unique?
Dr. Furuta: The 20 sites in CEGIR are able to collect a broad range of data from across the United States in a similar fashion. For a rare disease, this is unique to be able to do. In addition, the very collaborative nature of our investigators, research colleagues, and patient advocates makes us a unique research consortium. Finally, the numerous samples we have collected have been used to identify potential therapeutic targets for basic mechanistic studies.
How will this study contribute to clinical trial readiness?
Dr. Furuta: The collection of a large amount of data from children and adults across the nation makes for a rich database of patients with EGIDs that will allow us to understand what happens to patients over time and how best to measure it. We have been able to contribute to this knowledge in several ways that are supporting studies presently.
What has it been like for you to participate in this study?
Sandstrom: It has been pretty easy to participate in the study as they are only collecting samples whenever I need something done.
What are the successes and challenges of this study?
Dr. Furuta: We have been productive in our work with many primary publications in high-impact journals and a successful career enhancement core that is preparing the next generation of investigators. For a large network of people to work together effectively requires an excellent communication system and cooperation amongst many. We feel fortunate to have both of these, but it takes a lot of work by many. The change in staffing always creates challenges to maintain continuity in our studies.
What excites you about this study?
Sandstrom: They are trying to find out what causes EOE and hopefully finding a cure for it.
How will this study impact patients, now or in the future?
Dr. Furuta: The clinical outcome metrics, development of diagnostic guidelines, and identification of potential therapeutic targets will shape the field in the immediate future and for years to come. These findings are setting the foundation for rare disease research that many will benefit from in the research setting as well as clinical care.
How could this study impact your life?
Sandstrom: This study could impact my life by hopefully finding a cure so other children won't have to go through all the procedures I have had to do.
What is the status of this study, and what’s next?
Dr. Furuta: We continue to enroll patients who suffer from these rare diseases and are focusing on follow-up over time. We will be submitting our renewal application this year so we can hopefully continue to perform cutting-edge research for another five years.
“Outcome Measures for Eosinophilic Gastrointestinal Diseases across Ages (OMEGA)” is currently recruiting. Learn more about the study and how to join.
The Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). CEGIR is funded under grant number U54AI117804 as a collaboration between NCATS, the National Institute of Allergy and Infectious Diseases (NIAID), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).