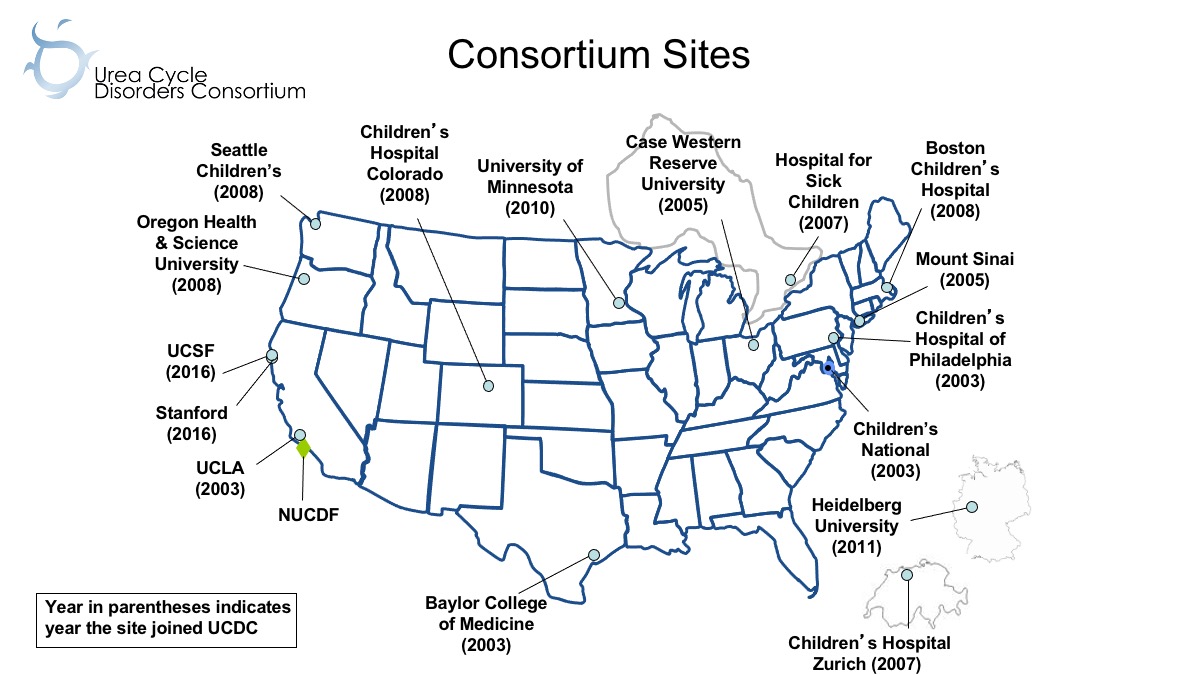
The Urea Cycle Disorders Consortium (UCDC), now in its fourth cycle of funding, is one of the original Rare Diseases Clinical Research Network (RDCRN) consortia. When originally established, the UCDC had five sites; it is now an international network of 16 academic centers (13 in the US, one in Canada, and two in Europe) with 38 faculty investigators and 25 research staff members providing state-of-the-art care and conducting innovative clinical research in urea cycle disorders (UCD).
Such exponential growth was possible not only through continued funding from the NIH, but also because of funding from philanthropic sources, including the Mary Alice and Thomas O'Malley Family Foundation, the Kettering Fund, the Rottenberg family, the Dietmar-Hopp Foundation, and the National Urea Cycle Disorders Foundation (NUCDF).
The UCDC focuses on the eight related disorders that are caused by deficiency in one of the six enzymes and two membrane transporters essential for urea biosynthesis: N-acetylglutamate synthase deficiency (NAGSD); carbamyl phosphate synthase 1 deficiency (CPS1D); ornithine transcarbamylase deficiency (OTCD); argininosuccinate synthase deficiency; (ASSD, citrullinemia); argininosuccinate lyase deficiency (ASLD, argininosuccinic aciduria); arginase deficiency (ARGD, argininemia); hyperornithinemia, hyperammonemia, homocitrullinuria (HHH) syndrome; and citrullinemia type II (CITRD).
Overall Goals of the Consortium
The UCDC’s major goals are to:
- Advance the understanding of the pathophysiology of UCD through collaborative clinical research;
- Nurture the development of the next generation of rare disease researchers;
- Identify promising new approaches to UCD care; and
- Disseminate knowledge and improve the care of UCD by providing ready access to information.
Establishing the UCDC
The original five UCDC site PIs had established relationships from serving on the NUCDF scientific advisory committee and through working on an early UCD treatment protocol for the use of phenylbutyrate. This collegial collaboration was the solid foundation for the UCDC’s original RDCRN grant application. Once established, the UCDC sought to enhance recruitment and enrollment in its Longitudinal Study (LS) by expanding geographic reach in order to serve as many participants as possible. To achieve this goal, the UCDC has expanded the number of sites and investigators through philanthropic funding.
Current Research Projects
The UCDC has initiated 14 major clinical research protocols, 14 pilot studies, and 26 career development projects during its 18-year funding period. In addition to several protocols continuing from previous grant cycles, current UCDC research projects include:
Project 1: Longitudinal Study of Urea Cycle Disorders
The Longitudinal Study (LS, 5101) is essential to the overall goals of the UCDC and is the basis of its research mission to address questions of pathophysiology and morbidity/mortality, as well as other outcomes of UCD including growth and development, metabolic status, nutritional status, cognitive function, treatment effects, pregnancy outcomes of affected mothers and their children, late effects and co-morbidities, and quality of life/mental health status. The LS not only furnishes the clinical data to explore these issues but also enables the identification of critical biomarkers that predict outcome and response to treatment, serving as a basis for clinical trial readiness and experimental therapeutics. Critical to advancement in this group of rare diseases, the UCDC has successfully enrolled, classified, and characterized a large patient cohort. The LS data has been used to expand knowledge of the natural history of UCD.
As of October 25, 2021, 907 participants have enrolled in the LS since the study was activated in February 2006, with a retention rate of 81% bolstered by use of remote consenting and data collection. Thirty-four publications have been written to date using LS data and several subsequent UCDC protocols have been powered by LS data. The LS data was also used to generate additional funding for the UCDC through a PCORI-funded study, which used LS data and additional data collected to compare liver transplant outcomes and medical management, and a post-market surveillance study for Carbaglu©, used to treat NAGS. Six pharmaceutical companies have used LS data to inform their clinical trials.
Project 2: Incidence and Prevalence of Seizures in Urea Cycle Disorders
Epilepsy has been considered an infrequent manifestation in UCD. However, we found frequent occurrence of sub-clinical electrographic seizures (ES; seizures detected on EEG without accompanying clinical manifestations) during acute hyperammonemic (HA) episodes in patients, especially neonates, enrolled in the LS. This new finding raised the question of whether seizures play an important role in the pathophysiology of neurocognitive deficits in UCD patients and/or can represent a biomarker that correlates with brain damage in these disorders. In this project (5119), we are systematically studying the scope and role of ES in our LS UCD population, characterizing the onset, frequency, duration, localization (focal or generalized) EEG background features, and biochemical and clinical correlates, as well as any association of ES or EEG background with the subsequent development of epilepsy.
Project 3: Noninvasive Biomarkers of Hepatic Fibrosis
Hepatocellular injury, hepatic fibrosis, and cirrhosis leading to liver failure have been described in a subset of patients with UCD. There are reports of abnormal liver function during acute episodes of hyperammonemia in several disorders, including OTCD. There are single case reports of hepatocellular carcinoma in UCD. Common histopathologic findings in the liver of individuals with UCD include hepatocyte enlargement, increased glycogen deposition, and steatosis. However, there currently are no recommendations for long-term surveillance for chronic hepatic complications. Although serum aminotransferases and markers of synthetic function are used to monitor hepatic disease, the sensitivity of such assays to detect progression of liver disease is low.
In this project (5120), we will use non-invasive tools and new biomarkers to assess the extent and prevalence of liver disease in a large cohort of individuals with UCD. In addition, we will histopathologically score fibrosis stage and steatosis grade, and quantify glycogen content in hepatic explants and biopsies from two UCDC liver transplant centers to determine the prevalence of these complications in UCD (5122). Overall, this will be the largest, most comprehensive study of liver disease in UCD. This study will inform our understanding of the natural history of liver disease in UCD, provide the basis for the development of new biomarkers for assessing liver disease in UCD, and validate endpoints that could be used in the future to assess efficacy of interventions for the prevention and/or therapy of liver disease in UCD.
Pilot/Feasibility Core
The Pilot/Feasibility Core serves as an “incubator” to nurture innovative approaches to diagnosis and management of UCD. The Core affords an essential link between patient needs—as articulated by clinicians and patient advocacy group representatives—and novel research proposals that may be, on occasion, high risk.
Fourteen pilot projects have been funded with NIH support to date. The pilot program has been very successful as demonstrated by:
- 10 publications resulting from pilot projects
- NIH R01 funding for an interventional clinical trial of N-carbamylglutamate in UCD
- NIH R01 for testing Nitric Oxide (NO) stimulation in UCD
- NIH R01 for dissecting the link between ureagenesis and hepatic glycogen metabolism
- Two pilot projects evolved to become major projects in the next grant cycle
- An industry-sponsored trial for a urease inhibitor
Career Enhancement Core
To date, the UCDC has funded 26 trainees/early-career investigators from eight UCDC sites. To enhance the number of trainees, the UCDC supplemented the NIH funds with career enhancement funds from the NUCDF and the O’Malley Family Foundation. Overall, the 26 CEC trainees have been very productive, collectively authoring more than 400 publications since 2004. Trainees have gone on to secure additional funding, lead major projects, and assume leadership roles in the UCDC.
Patient Impact
Partnership with Patient Advocacy Groups
The patient perspective is the major driver in the focus and activities of the UCDC. The UCDC’s experience has shown that collaboration with volunteer health organizations/patient advocacy groups is essential at all levels of planning and research prioritization.
The NUCDF has been an integral partner and collaborator in the UCDC since its inception. The executive director of NUCDF serves as a PI in our leadership and administrative core, as well as a member of the Executive Committee involved in oversight, direction, and strategic planning for the UCDC. NUCDF collaborates on study design, protocol development, informed consent, website content, recruitment, and the training program. NUCDF disseminates research findings to the UCD community to increase patient/caregiver/clinician knowledge and translation to patient care to improve outcomes.
Improving Care
The LS has enrolled participants for 15 years and has produced more than 34 publications, expanding our understanding of UCD. The resulting publications are used as guidance by metabolic geneticists treating UCD patients, leading to more standardized care and better outcomes. Through the RDCRN/UCDC public website, physicians can easily determine who to contact if they need to consult about a UCD patient.
Training Clinicians
Through the UCDC training program, patients now have greater access to well-trained clinicians who are accustomed to working with patient advocates. This partnership with patient advocates assures patients that their voices are being heard and incorporated in UCD research.
New Research Directions and Goals
With each grant cycle of NIH funding through the RDCRN, we have made sequential progress in understanding UCD. Lessons learned from pilot or major studies in one cycle inform new studies in the next cycle; both of the current new projects described here are examples of this process. We have identified new questions that we hope to answer through the LS based on data we have collected over the last 15 years.
For example, how should we manage late-onset female heterozygotes, how does UCD impact pregnancy outcomes, and what are the long-term co-morbidities associated with surviving UCD into adulthood? We have learned that it is critical to develop therapies and initiate treatment early. We have identified long-term outcomes that we didn’t expect. We learned that UCD management is more complicated than keeping ammonia levels down, and that there is a need to train adult metabolic physicians. Having partnerships with philanthropic foundations and biotech companies with a shared interest in the disorder-specific patient population has been mutually beneficial in promoting new therapies for UCD.
RDCRN’s Impact on UCDC
The UCDC would not have been able to grow and develop over the course of 18 years without NIH funding and as part of the RDCRN. RDCRN infrastructure support has been crucial in developing a robust data collection system. Regulatory and study monitoring support has helped to ensure the quality of the UCDC datasets. Patients with UCD and their families have greater confidence in participating in studies that are part of the NIH-funded RDCRN. The UCDC has made many advancements and developed innovative ways to conduct rare disease research, which we hope benefit RDCRN as well. The UCDC-RDCRN partnership has been a mutually beneficial collaboration.
How will UCD ultimately be cured?
While we strive for a cure, our immediate goal is to improve the quality of life for patients with UCD by translating research findings to medical management and accelerating new treatments. In collaboration with NUCDF, the UCDC has interacted with more than 20 pharmaceutical/bio-tech companies developing drugs for UCD. With the cooperation of the UCDC, two drugs have been brought to market and two more are in clinical trials, including a gene therapy. LS data helps pharma/bio-tech companies learn more about the disorders and assists in targeting their therapies. The UCDC gives companies a point of contact and helps make connections for clinical trials and find experts with whom to consult. Collaboration between industry and academic research accelerates the drug development process and brings more therapies to market.
The Urea Cycle Disorders Consortium (UCDC) is part of the Rare Diseases Clinical Research Network (RDCRN), which is funded by the National Institutes of Health (NIH) and led by the National Center for Advancing Translational Sciences (NCATS) through its Division of Rare Diseases Research Innovation (DRDRI). UCDC is funded under grant number U54HD061221 as a collaboration between NCATS, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).






