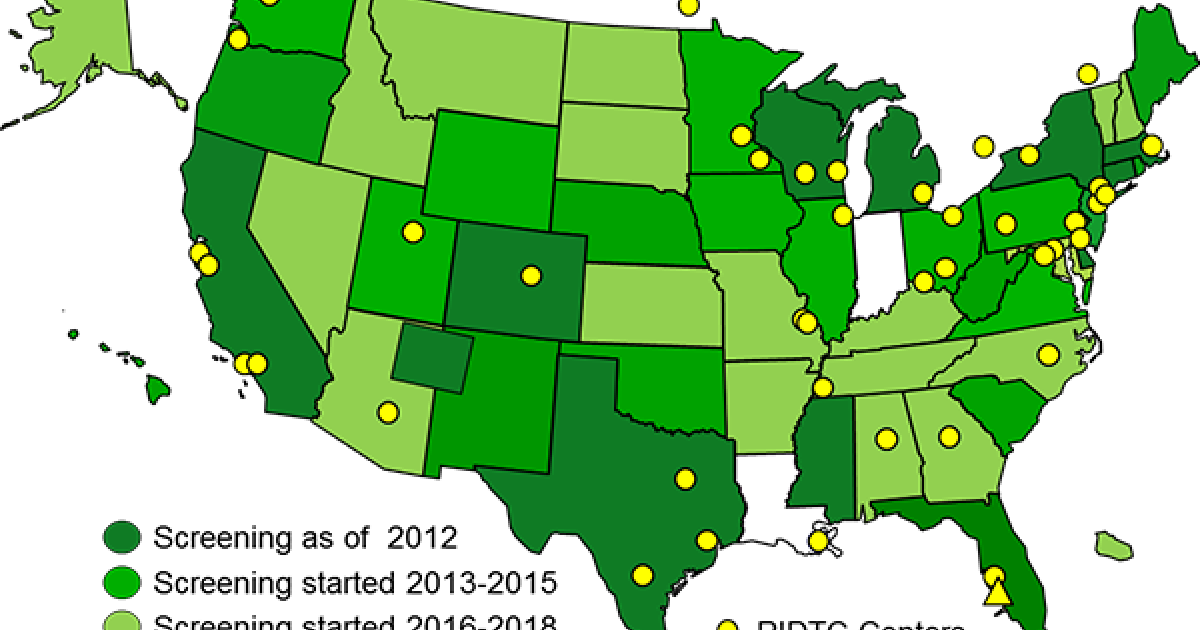
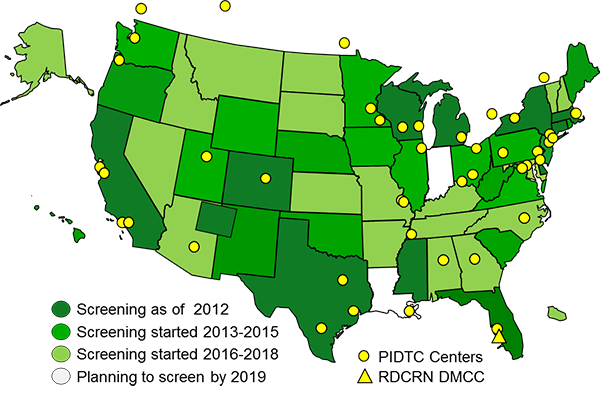
The Primary Immune Deficiency Treatment Consortium (PIDTC) is a part of the Rare Diseases Clinical Research Network (RDCRN) funded by the (National Institute of Allergy and Infectious Diseases (NIAID) and the Office of Rare Diseases Research (ORDR), National Center for Advancing Clinical and Translational Sciences (NCATS).
Major Goals and Diseases of PIDTC:
- Conduct multi-center studies of Primary Immune Deficiency (PID) diseases, organized into four Projects: Severe Combined Immunodeficiency (SCID), Chronic Granulomatous Disease (CGD), Wiskott-Aldrich Syndrome (WAS) and Primary Immune Regulatory Disorders (PIRD). The goals are to expand understanding and improve treatments and outcomes for patients with rare PIDs.
- Recognize, train and encourage new leaders in PID to foster the growth of future innovative research.
- Fund timely, innovative research through the Pilot/Feasibility Core.
- Provide Career Enhancement for new PID investigators.
- Engage Patient Advocacy Groups to share information between patients, parents, clinicians and scientists about up-to-date approaches to PID.
- Learn from and contribute to Rare Disease Research by collaborating with RDCRN partners.
Severe Combined Immunodeficiency (SCID)
SCID Studies
- Protocol 6901/6902: A Prospective Natural History Study of SCID/ A Retrospective and Cross-Sectional Analysis of SCID since January 1, 1968 (opened 2010; will close Aug 2019)
- Protocol 6907 SCID (proposed for Sep 2019 opening): 6901/6902 merged to continue as: SCID Prospective and Longitudinal Study of Genotypes, Management and Outcomes
The goal of the SCID studies is to advance the understanding, diagnosis, management, and treatment of SCID to define how best to achieve survival and full immune reconstitution after allogeneic hematopoietic cell transplant (HCT) or gene therapy. Results to date include demonstration that the specific genotype (from over 20 causative loci) plays a major role in outcome. Thus, it is vital to ascertain the underlying genetic cause in all patients. 279 patients have been enrolled to date in a prospective study and 741 in a retrospective/cross-sectional study. While most infants have known genotypes, those without a gene diagnosis will be studied with deep sequencing and mechanistic studies. These cohorts will be combined for future longitudinal studies with continuing enrollment of new patients. SCID has undergone a major change in presentation with newborn screening, now nearly universal in the USA and in place in several Canadian provinces.
Chronic Granulomatous Disease (CGD)
CGD Studies
- Protocol 6903: Analysis of Patients Treated for CGD Since January 1, 1995 (opened Jan 2014; will close Aug 2019)
- Protocol 6908 CGD (proposed for Sep 2019 opening): CGD: Determinants of Auto-Inflammation and Complications following Hematopoietic Stem Cell Transplant
The goal of the CGD studies is to define optimized curative transplant strategies and to understand and treat auto-inflammatory disease pre- and post-allogeneic HCT or gene therapy, a previously underestimated component of the disorder. A novel finding has been that successful HCT alleviated not only susceptibility to infection, but also auto-inflammatory complications. 379 patients have been enrolled to date.
Wiskott-Aldrich Syndrome (WAS)
WAS Study
- Protocol 6904: Analysis of Patients Treated for WAS since January 1, 1990 (opened Oct 2013; will close Aug 2019)
The goal of the WAS study has been to define the critical clinical factors and biologic markers that predict survival and other outcomes of children with WAS following HCT or medical therapies and to characterize the long-term outcomes. The overall study closed to new enrollment in September 2018, with publications to include outcomes and quality of life in 129 long-term survivors who received HCT after 2005.
Primary Immune Regulatory Disorders (PIRD)
PIRD Study
- Protocol 6906 (proposed for Sep 2019 opening): PIRD Clinical Presentations, Treatments and Outcomes
The goal of the PIRD study is to define natural history and long-term outcomes in PIRD following treatment with HCT, biologics and small molecules, while also providing basic insights into mechanisms of disease pathogenesis and therapeutic responsiveness.
Pilot/Feasibility Core: This core is responsible for soliciting, reviewing and overseeing the Pilot Projects awarded each year. Proposals with high scientific merit and potential to advance the field in an impactful manner are chosen. Pilot Projects may focus on PIDs already under study by the PIDTC or disorders under consideration for potential addition to the PIDTC disease list. The PIDTC has awarded 7 Pilot Projects to date, each selected after competitive and stringent review and funded for 1-2 years.
Career Enhancement Core: This core encourages involvement in rare disease research at the level of fellows and early faculty, and to promote interactions with senior physician scientists active in the field. An Annual PIDTC Scientific Workshop and Education Day offer opportunities for networking and career advancement for PID researchers in the PIDTC as well as RDCRN investigators in allied fields. The PIDTC has granted 20 fellowship awards to date. Our awardees have continued to contribute to PID research; all remain in academic positions.
PIDTC Patient Advocacy Groups (PAGs): The PIDTC engages multiple immunodeficiency Patient Advocacy Groups (PAGs) – the Immune Deficiency Foundation (IDF), Jeffrey Modell Foundation (JMF), SCID Angels for Life Foundation, CGD Foundation and Wiskott-Aldrich Foundation – in every facet of our organization.
PI: Jennifer Puck, MD
Co-PI: Don Kohn, MD
Visit: https://www.rdcrn.org/pidtc
The PIDTC is part of the Rare Diseases Clinical Research Network (RDCRN). Supported by NCATS and NIAID