A message from Tiina K. Urv, PhD, program director, Office of Rare Diseases Research within the National Institutes of Health's National Center for Advancing Translational Sciences.
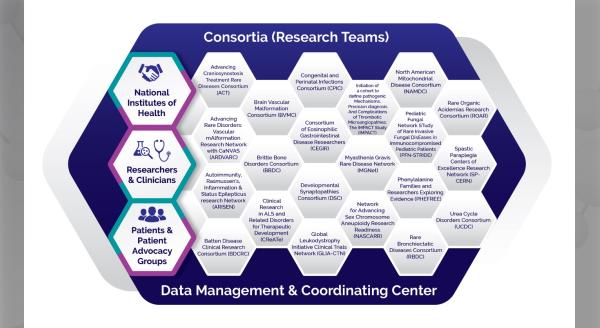
Today, we find ourselves facing challenges that would have been unimaginable when the Rare Diseases Clinical Research Network (RDCRN) awards for the fourth funding cycle were announced early last fall. At that time, we were anticipating changes in the network structure for the new funding cycle. We excitedly welcomed five new consortia to our network:
- Global Leukodystrophy Initiative Clinical Trials Network (GLIA-CTN)
- Congenital and Perinatal Infections Consortium (CPIC)
- Frontiers in Congenital Disorders of Glycosylation (FCDGC)
- Myasthenia Gravis Rare Disease Network (MGNET)
- Phenylalanine Families and Researchers Exploring Evidence (PHEFREE) Consortium
We also welcomed a new Data Management and Coordinating Center. The Coalition of Patient Advocacy Groups (CPAG), always a part of the RDCRN, was woven into the fabric of the network in a more formal manner.
And, most importantly, we sharpened the focus on the aims of the network for its fourth cycle.
The primary goal for the RDCRN has always been to advance the diagnosis, management, and treatment of rare diseases by promoting highly collaborative, multi-site, patient-centric, translational and clinical research.
While this is a laudable goal, we find ourselves, despite advances in our understanding of the causes and mechanisms of many diseases, in a place where effective treatments are still available for fewer than 5% of rare diseases. It has been estimated that 1 in 1,000 clinical trials are successful, and it is not unusual for a successful trial to take 10-15 years from the discovery phase. We continue to navigate a very long and risky road in our search for treatments for rare diseases.
Clinical Trial Readiness
These types of preparation help lessen risk and save time.
In this fourth funding cycle, we aim to lessen some of the risk by being well prepared when the opportunity of a potential treatment comes to light. This is “clinical trial readiness”—the ability to have everything in place, or “shovel ready,” when presented with a potential treatment.
Examples of activities that establish clinical trial readiness include natural history studies, which help us understand disease progression and provide an understanding of when and who to treat. Strong networks of clinicians experienced in conducting clinical trials allow for swift movement into new studies. Patient advocacy groups (PAGs) provide important insights into the needs and desires of patients and should be included early in the research process. Identification of biomarkers as well as sensitive and specific outcome measures allow accurate assessment of targeted treatments. These types of preparation help lessen risk and save time.
Sharing High Quality Data
A critical goal of the RDCRN is to advance rare disease research by freely sharing high-value data.
Another area of clinical trial readiness and one of primary importance in this fourth funding cycle is an emphasis on high quality data. Efforts to develop and monitor best practices for clinical and research data handling and use will be facilitated by our Data Management and Coordinating Center based at Cincinnati Children’s Hospital Medical Center.
Those efforts will include establishing standards for good data practices across the network, such as defining processes and technical standards; identifying security requirements; identifying, targeting and leveraging existing resources; and adhering to the FAIR (findable, accessible, interoperable, and re-useable) Data Principles.
A critical goal of the RDCRN is to advance rare disease research by freely sharing high-value data. Deidentified data collected within this network and housed within cloud services provisioned by NCATS will become a resource for the greater rare disease research community and will be made available to the scientific community, stakeholders, and other relevant partners in a timely and safe manner.
Cross-Network Collaborations
To make an impact, thinking outside of the “one disease at a time” box is imperative.
Finally, we will expand our vision beyond individual rare diseases or consortia and identify commonalities. This goal does not mean we will lessen the focus on what is unique about specific diseases. Rather, the intent is to augment the disease-specific findings with components that are shared across disorders.
By looking across the network and focusing on similarities like common symptoms, organ systems, or molecular pathways, we can learn from each other. With more than 7,000 rare diseases, moving forward one disease at a time would take a very long time. To make an impact, thinking outside of the “one disease at a time” box is imperative.
Responding to COVID-19
The RDCRN consortia have worked together collaboratively across the network to find answers—and if not answers, they found a supportive community.
During the current COVID-19 pandemic the RDCRN, as a whole, has risen to be greater than the sum of its parts. As the pandemic took hold, rare disease patients faced challenges coming from all directions. The RDCRN consortia have worked together collaboratively across the network to find answers—and if not answers, they found a supportive community.
The PAGs identified common challenges that they faced related to COVID-19 and shared strategies for addressing challenges and coping with a stressful time. The RDCRN consortia clinicians worked together with the PAGS to develop and disseminate trusted information related to COVID-19 and its impact on individuals with healthcare needs related to specific rare diseases. The RDCRN principal investigators collaboratively developed a survey for rare disease patients and their families on the multiple impacts of COVID‑19. The RDCRN principal investigators were able to come together as a team and develop the survey in weeks, not months. How? They are committed to their work, and they had their shovels ready!