A new gene is linked to primary ciliary dyskinesia (PCD), a rare disease that affects the airways. Using whole exome sequencing and bioinformatic analysis, researchers from the Genetic Disorders of Mucociliary Clearance Consortium (GDMCC) identified a variant in the CFAP57 gene that causes PCD, as published in the journal PLOS Genetics.
In patients with PCD, genetic mutations cause defects in the function of tiny, hair-like structures that line the airways. These structures, called cilia, carry mucus containing dust, bacteria, and other small particles out of the body. When cilia are unable to effectively clear these particles from the airway, this can lead to persistent infections of the lungs, ears, and sinuses.
“PCD is caused by over 40 identified genes, yet only 70 percent of clinically diagnosed individuals have a genetic explanation,” says study coauthor Amjad Horani, MD, director of the PCD and Rare Lung Disease Center at Washington University in St. Louis. “This means that a third of patients could have a genetic mutation that has not been associated with the disease.”
In order to learn more about how PCD is inherited and which genes are involved, the GDMCC assembled a team of clinical and basic science researchers within the motile cilia network. The team used a powerful tool to take on the diagnostic dilemmas of PCD—whole exome sequencing (WES).
This technique, which consists of sequencing all of the protein-coding regions of genes in a genome, is part of an ongoing effort from GDMCC researchers to explore possible genetic causes of PCD. Cumulatively, the team performed WES on more than 400 unrelated cases. Within this cohort, they found 99 cases with clinical features of PCD, but no obvious cilia defects.
Using a population sampling probability algorithm, the team analyzed this exome sequencing data. One of the subjects stood out—although they had been previously screened and found negative for genetic mutations associated with PCD, they also had typical symptoms of the disease.
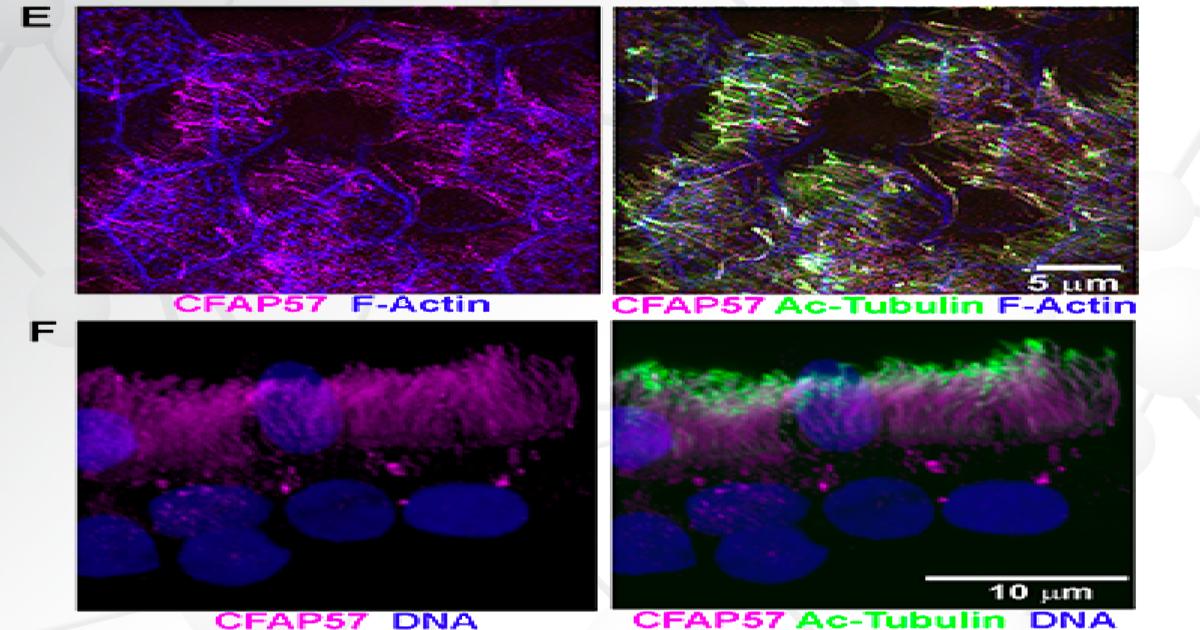
Researchers then sequenced the subject’s DNA and analyzed the resulting genotype data. After discovering a variant in the CFAP57 gene, the team investigated its effects by comparing the human nasal epithelial cells from the PCD subject with unrelated healthy controls. They found that the PCD cells showed reduced ciliary beat frequency and altered waveforms, demonstrating the crucial role of CFAP57 in ciliary motility.
As the first reported example of PCD caused by a mutation that likely only affects a subset of the inner dynein arms—important regulators of cilia motion—the team’s findings expand our understanding of the cilia assembly process.
“These results are only one piece of a big jigsaw puzzle,” says Horani. “Identifying CFAP57 as a structural component of motile cilia and associating it with disease prompts us to look for other components and associations of this pathway. Identifying these genetic contributors to disease will help us diagnose patients earlier to start treatment and slow the progression of lung disease.”
Read the full study in PLOS Genetics.
Learn more about the Genetic Disorders of Mucociliary Clearance Consortium (GDMCC).